
Story 2025/07/24
Galenica and the invisible work behind safe medications
What does it take to ensure that only safe and effective medications reach people in Switzerland? Galenica ensures that quality standards are met – from the place of manufacture all the way to the pharmacy or hospital. In this story, you’ll find out exactly how this works and what it has to do with underfloor heating.
“We have to guarantee that only approved and correctly stored medications get to the right places”, explains Remo Studer, Head of Quality Management and Responsible Person* at Galexis, Galenica’s logistics subsidiary. This involves meticulously coordinated processes that are continuously monitored and documented – including ensuring the traceability of each individual product.
Responsibility along the entire supply chain
The safety of medications in Switzerland is based on a fine-tuned interplay between different players: specialist bodies such as the authorisation authority Swissmedic and the cantonal regulatory authorities for therapeutic products, pharmaceutical manufacturers, marketing authorisation holders and, last but not least, companies like Galenica. As a leading player in the Swiss healthcare market, Galenica assumes responsibility along the entire supply chain: from procurement and storage to delivery and dispensing medications to patients – within its own pharmacy network for example.
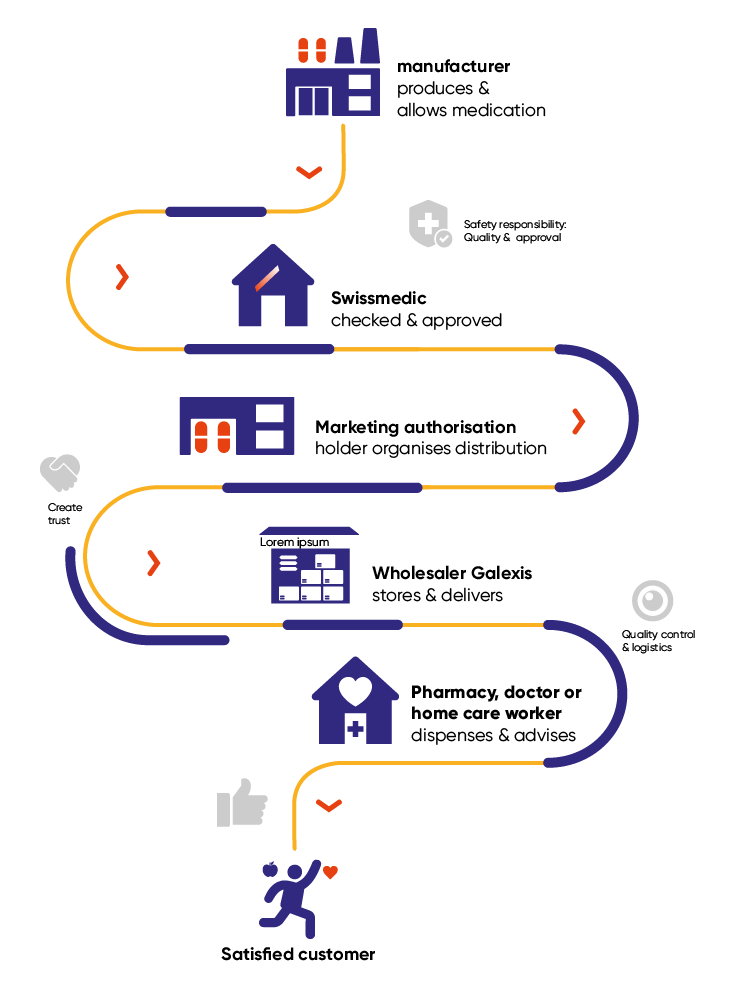
Quality from the outset
Quality assurance begins with the receipt of medications from marketing authorisation holders, such as pharma companies. Only medications that have been approved by Swissmedic and originate from approved suppliers are admitted into the warehouse. Each product is checked against the regulatory requirements by a qualified member of staff. “Although we can’t open boxes, we have to make sure that quality is guaranteed at all times – for example, by checking the vehicles that deliver the goods”, says Studer.

We continuously measure the temperature in every room and in every vehicle using sensors that immediately sound the alarm in the event of deviations.
Remo Studer, Head of Quality Management and Responsible Person at Galexis
Cold chain and transport: a logistical challenge
Medication safety does not end in the warehouse. It must also be ensured that temperature specifications – usually 15–25 °C or 2–8 °C depending on the type of medication – are strictly adhered to during transportation to customers. Remo Studer explains: “We continuously measure the temperature in every room and in every vehicle using sensors that immediately sound the alarm in the event of deviations.” Medication delivery vehicles are also equipped with underfloor heating to counteract temperature fluctuations, especially in winter. This ensures that conditions remain stable even in the face of up to 80 door openings per delivery run.
The ability to act quickly and purposefully in the event of problems
Galexis works closely with Swissmedic and the cantonal pharmacies, particularly where sensitive products such as narcotic substances are involved. This close collaboration ensures that all legal requirements are complied with – and that the system can respond quickly if necessary, such as in the event of recalls. “We know which medication has been delivered and where at all times”, stresses Studer. This makes it possible to act quickly and purposefully in the event of quality problems – a prerequisite for effective recall campaigns.
The production in Europe would help stabilise the supply and make it more independent.
Supply chains under pressure: why more production is needed in Europe
“We see a growing risk in international supply chains”, says Remo Studer. Many active pharmaceutical substances are now produced in Asia. This concentration brings with it dependencies and potential bottlenecks. “If several marketing authorisation holders have production at the same location and something happens there, it can affect entire product groups”, warns Studer. He therefore welcomes the growing political debate surrounding bringing the production of active substances back to Europe at an increasing rate: “This would help stabilise the supply and make it more independent.”
Responsibility for supply – today and tomorrow
Galenica has a duty not only to patients, but also to pharmacies, doctors, hospitals and care homes. Every day, the company works to ensure that medications arrive safely and are used correctly. This work includes strict quality controls, smooth logistics processes and a clear understanding of what the authorities require. Galenica will continue to adapt its processes in the future in order to prepare for new challenges and ensure a reliable supply of safe medications for the people of Switzerland going forward.
*Responsible Person is a term from Swiss pharmaceutical law. This person is responsible for ensuring compliance with the relevant pharmaceutical regulations in the manufacture, import, wholesale and export, foreign trade and approval of medicinal products.
Dr. Remo Studer
Motto: “You have to try the impossible to achieve the possible.” (Hermann Hesse)
- Head of Quality Management and Responsible Person at Galexis
- Member of the Galexis management team
- Swiss-certified pharmacist, doctorate in structural biology at the Paul Scherrer Institute and ETH Zurich




